Synchronize the path to regulatory approval
Comprehensive governance and data exchange that unlocks patient journeys before, during and after clinical trials
More trial, less error
With high error rates from legacy technologies, we believe the token is broken, limiting life sciences organizations from realizing the full potential of real-world data (RWD) in maximizing the investment in clinical trials. HealthVerity FLOW for Clinical Trials is the only patient journey software solution that can synchronize patients across de-identified, identifiable and investigator data, all in a fully governed, HIPAA-compliant and 21 CFR 11-certified environment. Powered by the IPGE approach, this end-to-end patient journey software solution optimizes trial insights throughout the clinical research stages, from recruitment and enrollment screening to external control arm and long-term follow-up studies.

Store, manage and report patient participation
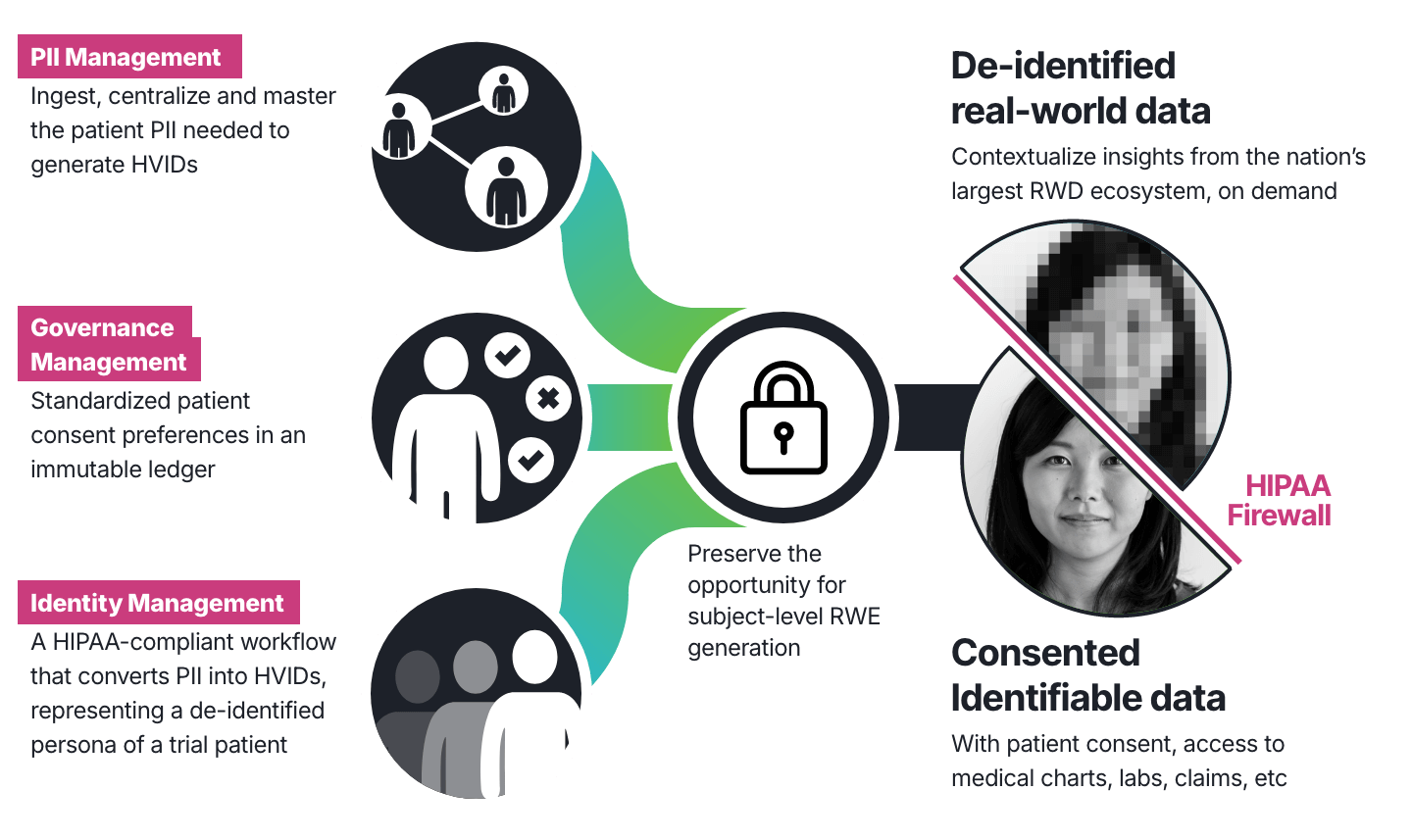
HealthVerity FLOW is ideally positioned to provide its clients with an enterprise view of key activities in a single phase II, III or IV trial or across the entire portfolio of clinical trials. By providing a dashboard view of enrollment shared with the application, HealthVerity FLOW can effectively store, manage and report on patient participation as a tokenized source of truth, efficiently manage consents and permissions throughout the duration of a trial, and provide an on-demand means to retrieve de-identified and identifiable data, often the same day, all in a HIPAA-compliant manner and with full data provenance.
The ultimate source of truth
To ensure the most accurate and data-driven outcomes, begin your clinical trial journey with HealthVerity Identity Manager, the industry’s most accurate solution for patient matching and identity resolution. Serving as the single source of truth for enrolled patients across the enterprise, HealthVerity offers a reliable platform to track and manage patient participation over time, while preserving the option to seamlessly retrieve patient-centric de-identified or identifiable patient data.
Built-in privacy and consent management
Confidently manage both identifiable and de-identified data, patient consents and user permissions throughout the study and beyond. This 21 CFR Part 11-certified solution fully integrates with eConsent systems in addition to offering a distributed webform for site-level data capture.
On-demand data discovery and delivery
Unlock patient journeys before, during and after clinical trials with on-demand real-world data discovery and delivery from the nation’s largest healthcare and consumer data ecosystem. Overlap your trial cohorts in real-time across the universe of RWD in HealthVerity Marketplace to explore comorbidities, biomarkers or important physician notes, all in a HIPAA-compliant manner. Just as easily retrieve a wide breadth of identifiable patient data for those who have given consent to further generate real-world evidence in combination with your investigator findings.
Discover the difference
Recommended for you
De-ID that’s economical,
not astronomical
A guide to optimizing real-world data: Electronic medical records
Zombie Data Explained
HealthVerity Marketplace
Drag and drop data sources to explore intersections

